Graphene is a new type of two-dimensional material, which has excellent mechanical properties comparable to that of carbon nanotubes. It also has excellent thermal physical properties such as low thermal expansion coefficient and high thermal conductivity, and its quantum effect is peculiar. Therefore, the combination of graphene and aluminum metal to prepare graphene aluminum-based composite materials will have excellent properties such as light weight, high strength, low thermal expansion and high thermal conductivity, and meet the needs of practical aerospace and other structural parts and microelectronic packaging fields.
Since the graphene monolayer structure was first stripped by Novoselov et al. in 2004, studies on graphene and its application properties have been extensively developed in various fields. Graphene is a crystal formed by sp2 hybrid carbon atoms having a thickness of only a single atomic layer and arranged in a two-dimensional hexagonal grid shape.
When an external mechanical force is applied, the carbon atom layer is bent and deformed to accommodate external forces without having to rearrange the carbon atoms, thus maintaining structural stability. When electrons in graphene move in a two-dimensional hexagonal grid, they do not scatter due to lattice defects or dopant atoms. Due to the strong interaction between atoms, even in the case of collision between surrounding carbon atoms at normal temperature, the interference of electrons in graphene is very small.
Graphene has many excellent properties. For example, the theoretically ideal single-layer graphene has a specific surface area of ​​2,630 m2/g and a thickness of only 0.35 nm. Ideally, electrons move faster on graphene than in general conductors. The speed of motion reached 1/300 of the speed of light; the tensile modulus and mechanical strength of graphene reached 1000 and 130 GPa, respectively, which is currently the highest known, more than 100 times that of steel.
In order to further explore these properties in various applications, the researchers developed a variety of synthetic pathways for the synthesis of graphene and graphene-based composites.
To date, graphene has been successfully compounded with inorganic nanostructures, organic crystals, polymers, metal organic frameworks, biomaterials, carbon nanotubes, etc., and in batteries, supercapacitors, fuel cells, photocatalysis, sensing The field of Raman enhancement has been extensively studied.
Preparation of graphenePreparation of graphene Since the earliest mechanical stripping method, various preparation methods have been developed, such as crystal epitaxy, chemical vapor deposition, liquid phase direct stripping, high temperature deoxidation and chemical reduction. Researchers in China have carried out research work on graphene preparation earlier. Chemical vapor deposition is a common method for preparing large-area graphene.
At present, most of the hydrocarbon gases (such as CH4, C2H2, C2H4, etc.) are used as precursors to provide carbon sources, and solid carbon aggregates can also be used to provide carbon sources. For example, Sun et al. use chemical vapor deposition to deposit polymer films on metal catalyst substrates. Above, high quality layer controllable graphene is prepared. Compared with chemical vapor deposition, plasma enhanced chemical vapor deposition can produce single layer graphene at lower deposition temperatures and shorter reaction times. Further, the crystal epitaxial growth method removes Si by heating single crystal 6H-SiC, thereby obtaining graphene epitaxially grown on the surface of SiC. However, the surface of the SiC crystal is restructured during high temperature, which makes the surface structure more complicated, so it is difficult to obtain graphene with a large area and uniform thickness.
However, the characteristics of solvothermal method for preparing high-quality graphene under high temperature and high pressure closed system are more and more concerned by researchers. Graphene nanoribbons having no defects and having a defined structure can be prepared by organic synthesis methods compared to other methods.
Unlike the above-described bottom-up synthesis method, the top-down method can increase graphene yield and is easy to prepare. Such as the simple chemical peeling method and graphite oxide reduction method, the latter has become the simplest method for laboratory preparation of graphene.
The solvent stripping method developed next is less toxic than the redox method and does not destroy the structure of graphene. In addition to the chemical reduction method, the graphite oxide can also be reduced to graphene by an electrochemical method, but the ratio of C to O atoms in the graphene prepared by the method is low. In addition, microwave methods are also used to prepare graphene.
First, graphite oxide was prepared by the modified Hummers method [12]. The experimental material was natural graphite (≥99.0%) having a particle size of about 43 μm. Graphite, NaNO3 (≥99.0%) and KMnO4 (≥99.5%) were prepared at a weight ratio of 2:1:6. Graphite and NaNO3 were added to concentrated sulfuric acid (≥95.0%) at 0 °C, and low temperature and medium temperature reactions were carried out at 10 and 35 °C for 2 h under the action of KMnO4. Then, deionized water was added to the intermediate temperature reaction product to continue the reaction, and the reaction temperature was controlled below 95 ° C for 30 min. Add 5% H2O2 solution to the high temperature reaction product to dissolve MnO2, stir the solution to golden brown, then wash it with 5% concentration of diluted hydrochloric acid and deionized water for 3 times, remove the filtered product, and dissolve it with sufficient deionized water. After 2 hours of ultrasonic treatment, a graphene oxide colloid was formed. The third aliquots of the colloids were heated to 60, 80, 98 ° C in a water bath, and continuously stirred. At the same time, excess N 2 H 4 ·H 2 O (≥80%) was added, and after 2 h of reaction, the mixture was washed and filtered, and the filtered product was dried to obtain graphene. The prepared graphene was analyzed by a polycrystalline X-ray diffractometer (Panaco, Netherlands, X'PertPRO type), and the scanning peak was observed by a scanning electron microscope (Japan Tsushima Co., Ltd., SSX-550). Surface morphology of graphene samples after gold.
Preparation of graphene-CuAdding a sufficient amount of 0.1 mol/L CuSO4 solution to the graphene oxide colloid prepared by the above method, stirring and mixing, heating to 98 °C in a water bath, then adding excess N2H4·H2O for 2 h, washing and filtering, drying to obtain graphene- Cu. The diffraction peaks of the obtained graphene-Cu samples were analyzed by polycrystalline X-ray diffractometer, and the surface morphology of the samples after gold spraying was observed by scanning electron microscopy.
Preparation of graphene aluminum matrix compositesThe 99.99% industrial pure aluminum ingot was heated and melted to 720 °C by an induction furnace, and the melt was stirred by a precision booster electric stirrer (Shanghai Pudong Physical Optical Instrument Factory, JJ-1 type). At the same time, the self-made graphene-Cu is continuously added. The weight ratio of graphene-Cu to aluminum block is 1:50. When the temperature of the aluminum liquid drops to 660 ° C, the stirring resistance is significantly increased, the stirrer is taken out, and the melt is cooled to room temperature. The hardness of the sample was measured using a 430/450 SVDTM Vickers hardness tester, and the surface morphology of the sample was observed by a scanning electron microscope, and the composition thereof was analyzed.
results and analysisStructure and distribution of graphene
Figure 1 is an XRD pattern of graphene prepared under different reduction temperatures. The diffraction peak at 2θ = 26.7° is a characteristic peak of graphite (002), which indicates that the sp3 hybridized carbon is reconverted into sp2 hybridized carbon. There is a relatively strong diffraction peak near 2θ=21° on the left side of the graphite characteristic peak (002). Since the oxidation treatment destroys part of the carbon six-membered ring, the graphite sheet contains a large amount of oxygen-containing functional groups, and the reducing agent does not completely reduce all oxygen-containing functional groups. Most of the destroyed carbon six-membered rings cannot be restored to their original appearance, and, after reduction, The graphite cannot be completely restored to the original sheet stacking structure. Therefore, there is a 2θ=21° diffraction peak on the X-ray diffraction pattern. Figure 2 is a SEM photograph of graphene prepared under different reduction temperatures. It can be observed from Fig. 2a that the graphene sheets are cross-aggregated together, and the sheet stacking structure of graphite is not observed. This indicates that the structure of the reduced product has changed after the oxidation and ultrasonic treatment of the graphite. The graphite sheet layer is changed from an ordered stack to an unordered stack. Residual oxygen-containing functional groups, graphite sheet structure defects and disordered stacking between graphite sheets resulted in a shift of the graphite characteristic diffraction peak (002) to the left and a new diffraction peak near 2θ=21°. The surface morphology of the graphene obtained by reduction at 60, 80, 98 °C is different, which indicates that the reduction temperature has an effect on the degree of agglomeration of graphene. As the reduction temperature increases, the agglomeration of the reduced product gradually decreases. When the reduction temperature is 98 °C, the graphene sheet has the smallest agglomeration, and it is the easiest to find the off-line graphene sheet.
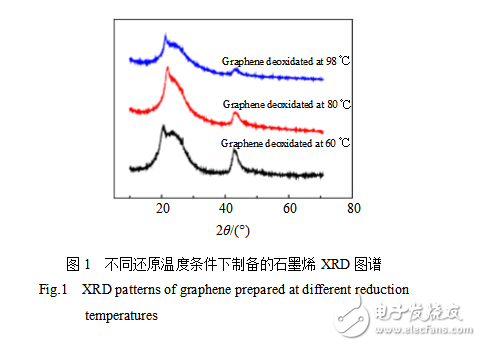
Comparing the three XRD curves in Fig. 1, the curves with a reduction temperature of 98 °C have peaks at 2θ=21° and 2θ=26.7°, which are less than 60 and 80 °C peaks at these two points, indicating that the graphite has a reduction temperature of 98 °C. The oxidation reaction and the reduction reaction of graphite oxide proceed more fully.
Figure 3 is an XRD pattern of graphite and graphene reduced at 98 °C. It can be seen from the figure that the XRD curve of graphene reduced at 98 °C is close to a straight line under the strong contrast of graphite (002) characteristic diffraction peaks. No obvious diffraction peaks were observed, indicating that the prepared graphene has reached the requirement for preparing graphene aluminum-based composite materials.
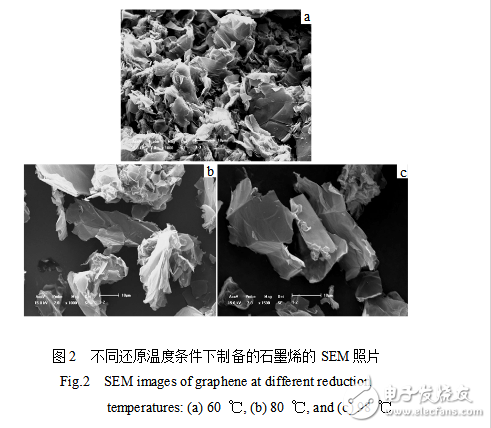
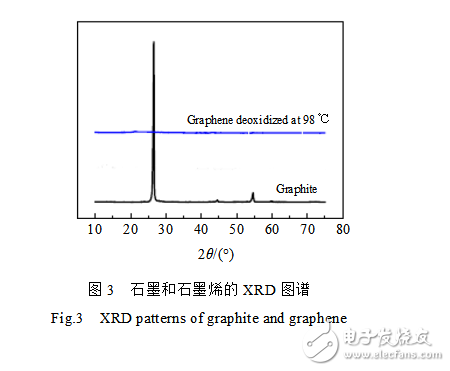
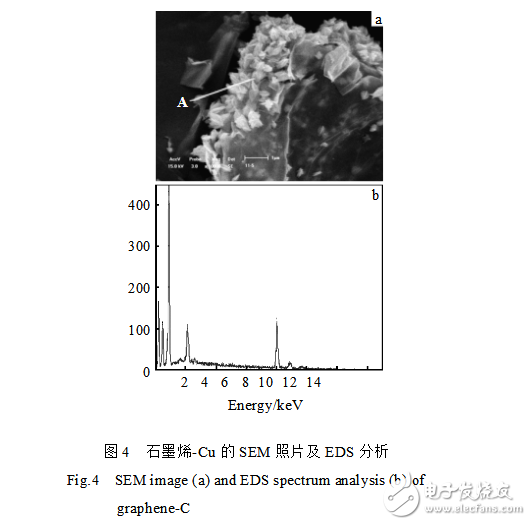
Figure 4 is a SEM photograph and energy spectrum analysis of graphene-Cu. As can be seen from Fig. 4a, some fine crystal particles are attached to the edge of the produced graphene. The energy spectrum analysis was performed on point A in Fig. 4a, and the results are shown in Fig. 4b. From the test results, it was found that the components of these fine particles contained copper and oxygen. Analysis of the XRD curve of Fig. 5 revealed that a large amount of copper simple substance and copper oxide existed in the sample, and it was confirmed that the composition of the small crystal grains in Fig. 4a was copper oxide and copper. Therefore, copper ions in copper sulfate are reduced to elemental copper and copper oxide and adsorbed on graphene.
The density of copper is 8.96 g/cm3, the density of copper oxide is affected by its structure, between 6.32 and 6.45 g/cm3, and the density of graphene can be referred to the density of graphite (2.21~2.26 g/cm3). The density of copper and copper oxide is much greater than the density of graphene. The copper and copper oxide particles are adsorbed onto the graphene, which increases the density of the graphene. Moreover, the copper atom and the aluminum matrix have good wettability, and thus the wettability of the graphene and the aluminum matrix to which the elemental copper and copper oxide particles are attached is also greatly improved.
Fiber Optic Components,Parts Of Fiber Optic Cable,Fibre Optic Connector,Parts Of Optical Fiber
Cixi Dani Plastic Products Co.,Ltd , https://www.dani-fiber-optic.com